A cornucopia of new bills; Canadians support end-of-life psilocybin therapy; and tempering the hype
Plus: Antidepressants may dampen psychedelics’ subjective effects and FDA group holds psychedelics conference
Happy Friday and welcome back to The Microdose, an independent journalism newsletter brought to you by the U.C. Berkeley Center for the Science of Psychedelics.
The State of Psychedelics: A cornucopia of new bills
By our count, at least 10 states have introduced new psychedelics-related legislation since the start of the year, including a handful of new bills in recent days.
In Alaska, senators Forrest Dunbar (D) and Elvi Gray-Jackson (D) introduced Senate Bill 166, which proposes the state form a Mental Health and Psychedelic Medicine Task Force to scope out the possibilities for legalizing “psychedelic medicines” at the state level. The task force would also recommend insurance and licensing requirements that would go into effect if the U.S. Food and Drug Administration approves any psychedelic substance as medicine, or if the U.S. Drug Enforcement Administration were to reschedule any of these substances into a less restricted category.
Last week, Senator Chris Lee (D) of Hawaii introduced Senate Bill 3019. The bill, and its companion bill HB 2631, would allow people authorized by a physician to receive psilocybin therapy, and to protect them from existing state law prohibiting psilocybin. Last February, the Hawaiian legislature passed a bill forming a Therapeutic Psilocybin Working Group to investigate the use of psilocybin therapy. Senator Lee told Marijuana Moment this week that the new bill is a direct result of the group’s work over the last year.
On Tuesday, Missouri’s Veterans Committee held a hearing to discuss House Bill 1830, which would allow, at the state level, people with PTSD, depression, substance use disorders, or who are in palliative care to access up to 150 mg of ‘psilocybin analyte’ annually. Psilocybin analyte refers to the amount of active psilocybin in mushroom matter, as opposed to just the total weight of the mushrooms themselves. For reference, people visiting psilocybin service centers in Oregon are allowed to consume up to 50 mg of psilocybin analyte in a single visit; 150 mg would provide enough psilocybin for several medium doses of the drug. The substance remains illegal federally.
In Arizona, members of the House Military Affairs and Public Safety committee voted to advance House Bill 2105, which extends the spending deadline for a 2023 bill that appropriated $5 million for psilocybin research grants. If Bill 2105 passes, researchers can spend that money over several years rather than having to spend it by July 1, which would not be feasible.
Survey: Canadians support end-of-life psilocybin therapy
In 2022, the Canadian government introduced new procedures allowing medical professionals to request access to psychedelic-assisted therapy on behalf of their patients. Shortly after those changes were introduced, a group of researchers from Quebec surveyed 2,800 Canadians about their attitudes towards psilocybin-assisted therapy, or PAT. The results, published in Palliative Medicine, suggest Canadians are largely supportive of PAT in helping treat existential distress at the end of people’s lives: 79.3% agreed that PAT was “a reasonable medical choice” for such patients, and 84.8% said they thought the Canadian government should cover the cost of that treatment. The researchers also asked respondents about which care scenarios they’d consider for their own end-of-life care. While a majority of people said they had a favorable view of undergoing PAT with a certified healthcare professional, most said they had an unfavorable view of using psilocybin with facilitators who aren’t therapists, or of using the substance without supervision. The authors acknowledge that respondents who answered the survey may not be representative of the general population, but that the high level of support for end-of-life PAT “may help stakeholders mobilize resources to address barriers and challenges” for patients trying to access therapy.
Want the latest psychedelics news? Subscribe! (It’s free!)
Tempering the hype
This week, Harvard Law School’s Petrie-Flom Center for Health Law Policy, Biotechnology, and Bioethics launched a new symposium on critical psychedelic studies. Last fall, Harvard announced a $16 million grant for the Study of Psychedelics in Society and Culture. In the introductory post for the new symposium, organizer and psychedelics scholar Neşe Devenot wrote about the growing number of former psychedelics insiders who have become increasingly critical of the hype surrounding psychedelics, and concerned about the “ethical and political impact of psychedelic medicalization and its capitalistic roots.” The symposium’s first two pieces address the potential for psychedelic harm in clinical settings, and psychedelic evangelism in the current research environment. In the latter piece, psychology researcher Patric Plesa discusses how recent psychedelic research focuses on individual therapeutic intervention and how this contrasts with many Indigenous healing traditions, which administer psychedelics in group settings. “Individual psychedelic interventions fail to address the possibility for communal meaning-making and solidarity that could be actualized with group psychedelic-assisted therapy,” Plesa writes.
FDA group holds psychedelics conference
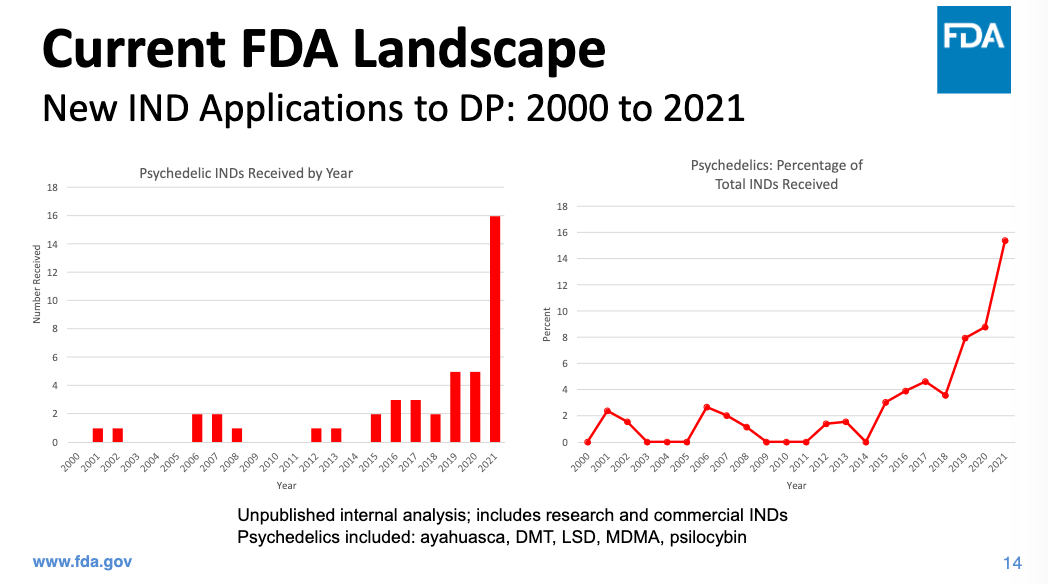
The Reagan-Udall Foundation, a non-profit created to advance the work of the U.S. Food and Drug Administration, held a two-day conference this week on current psychedelic research and clinical study design. Speakers included FDA representatives and researchers at universities as well as psychedelic companies like Compass and MindMed. In her presentation, the FDA’s Office of Neuroscience director Tiffany Farchione presented FDA data showing that the number of Investigational New Drug applications for psychedelic drugs had increased significantly in the last few years; while there were between 0 and 3 such applications submitted each year between 2000 and 2016, there were 16 in 2021.
Other sessions discussed ongoing issues in psychedelic research, including how to assess the contribution of participants’ expectations in the study outcomes; how long positive effects from psychedelic-assisted therapy might last; and how to design randomized controlled trials in which participants truly can’t tell whether they’re part of an experimental group that received a psychedelic versus a control group that received a placebo.
Bon Appetit explores the world of illegal mushroom chocolates.
Psychedelic educators are getting banned on social media, which provides “a potent example of our completely haphazard, inconsistent approach to what digital discourse is permitted around substance and drug education,” cannabis consultant Robert Johnson writes in Rolling Stone.
The Boston Globe breaks down the top 2023 donors to Massachusetts’ new ballot initiative. (The top donor, contributing $1 million, was All One God Faith Inc., or Dr. Bronner’s Magic Soaps.)
Psychonauts often recommend the anxiety-reducing drug alprazolam (Xanax) to mitigate the effects of a bad trip, but does that actually work? Salon investigates.
Harvard Divinity School is holding a conference on February 17 devoted to “how psychedelics and spirituality intersect differently across cultures, contexts, and traditions.”
You’re all caught up! Have a great weekend. We’ll be back in your inbox on Monday with a new issue of 5 Questions.
If you know anyone who might like the latest on psychedelics in their inbox, feel free to forward this to them, or click below.
Got tips? Email us at themicrodose@berkeley.edu.