Closed-door MAHA summit discusses psychedelics, Maryland task force recommends psilocybin program, and New Oregon psilocybin rules
Plus: Synthetic 5-MeO-DMT reduces depression scores, and Psychedelic use and alcohol use disorder
Happy Friday and welcome back to The Microdose, an independent journalism newsletter brought to you by the U.C. Berkeley Center for the Science of Psychedelics.
Closed-door MAHA summit discusses psychedelics
On Wednesday, Secretary of Health and Human Services Robert F. Kennedy Jr. and his “Make America Healthy Again” allies held a day-long summit at the Waldorf Astoria hotel in Washington DC. The speaker list included a who’s who of government officials including Vice President JD Vance and Food and Drug Administration commissioner Marty Makary. The summit also included industry leaders and medical technology, or “medtech” influencers such as longevity entrepreneur Bryan Johnson, who, earlier this week, livetweeted his trip after taking nearly five grams of dried Psilocybe cubensis as a “longevity therapy.”
The summit was closed to the media but speakers covered a wide variety of topics such as the future of the FDA and NIH. Psychedelics were also on the agenda at the summit in a session with Christian Angermayer, co-founder of psychedelic company atai (now AtaiBeckley) and an early funder of Compass. Angermayer was joined by Matt Zorn, an attorney who has sued the federal government over cannabis and psychedelic drug reform issues, and accepted a position as Deputy General Counsel at Health and Human Services in May. Some have called him the Trump administration’s “psychedelics czar.” The conversation was titled “Psychedelic Medicine: The Next Frontier in Mental Health.”
While little was reported from inside the summit due to the ban on media, attendees posted snippets on social media sites.

Maryland task force recommends psilocybin program
In 2024, the Maryland legislature passed a bill establishing a 19-person task force to study “natural psychedelic substances,” including psilocybin, DMT, and mescaline (excluding peyote). Recently, that task force released their findings in a detailed 373-page report, along with a set of recommendations.
Ultimately, the group recommended starting with psilocybin, and gradually rolling out psychedelic reforms in three stages. First, establish regulatory infrastructure like advisory boards, facilitator training, law enforcement training, and testing labs for a psilocybin program. Next, launch a program that includes medical oversight of psilocybin use before potentially allowing commercial sales, and expansion to other psychedelics.
The task force’s goals, they write, are to “ensure responsible and gradual expansion of access while maintaining capacity to identify and respond to emerging issues swiftly” — and part of that, it seems, is not waiting for federal agencies to take the lead. The group “does not support delaying state action pending future federal FDA approval,” the report states, in bold.
<break>
Want the latest psychedelics news? Subscribe! (It’s free!)
<break>
New Oregon psilocybin rules, including psilocin reporting
Last Thursday, Oregon Psilocybin Services announced they had adopted amendments to the Oregon Psilocybin Services Act, which will go into effect January 1, 2026. Many of these changes are clearly a response to issues facilitators and clients have noted along the way. The new rules represent efforts to make the nation’s first state-regulated psilocybin program safer and more equitable.
Some of the rules were mandated through the passage of House Bill 2387, also known as the Oregon Psilocybin Services Program Improvement Bill, earlier this year. The bill shields health professionals who facilitate psilocybin sessions from disciplinary action by state professional regulatory boards such as the Oregon Medical Board, and requires that all psilocybin products be labeled with their psilocin content in addition to their psilocybin content. (A quick primer on psilocin: psilocybin is the prodrug of psilocin; when a human ingests psilocybin, it’s metabolized into psilocin. Mushrooms can contain both psilocybin and psilocin so knowing the quantity of psilocybin alone is not sufficient to predict the strength of a dose.)
The newly adopted amendments also include changes mandated by Senate Bill 907, also passed earlier this year, requiring all licensed psilocybin manufacturing facilities and license applicants to submit a notarized consent form from the owner of the property they operate from acknowledging that they approve.
Other changes include allowing psilocybin facilitator trainees to transfer hours between programs, should their program shut down, and requiring service centers to include how many clients opted-out of data sharing in their quarterly reports to the state.
Synthetic 5-MeO-DMT reduces depression scores
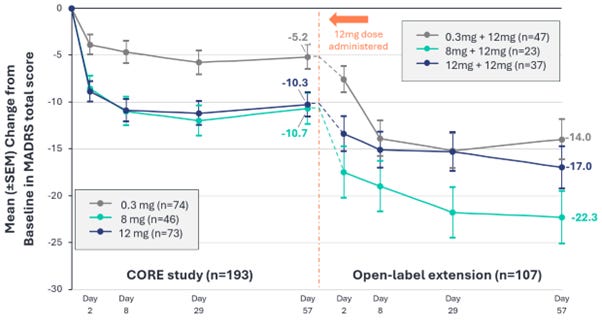
On Monday, pharmaceutical company AtaiBeckley announced preliminary results from an open-label extension following its Phase 2b clinical trial using BPL-003, a synthetic 5-MeO-DMT nasal spray, for treatment-resistant depression. In the initial clinical trial, the results of which were released in July, nearly 200 participants received 12mg, 8mg, or 0.3mg (placebo) doses of BPL-003. Researchers took baseline measures of participants’ scores on the MADRS, a standard depression questionnaire, and again the following day, the following week, four weeks later, and eight weeks later. The company reported that participants who received the largest dose showed, on average, an 11-point lower MADRS score four weeks after receiving BPL-003, while those in the placebo group showed a 6-point reduction. (A lower MADRS score indicates fewer depression symptoms.) MADRS scores of those who received 8mg of BPL-003 were, on average, 12 points lower, similar to that of the 12mg group, suggesting that the slightly smaller dose “may be sufficient to achieve therapeutic benefit,” the company’s press release says.
The study’s extension entailed giving over 100 participants from the Phase 2b trial a 12mg dose of BPL-003 8 weeks after their initial dose, then tracking their MADRS scores the next day, and then again at one-week, one-month, and 8-week timepoints. Unlike the initial Phase 2b study, where both participants and researchers were blinded to which dose participants received, this extension was open-label: all involved knew participants were receiving a 12mg dose of BPL-003. All three groups showed lower depression scores after receiving the drug, and those effects persisted to the 8 week mark.
The results follow a flurry of recent headlines from the company - chief among them that atai and Beckley Psytech recently merged, and the U.S. Food and Drug Administration granted BPL-003 breakthrough therapy status designation. The company says it has a meeting scheduled with the FDA to discuss the design of its Phase 3 clinical trial program, which could begin as soon as the second quarter of next year.
Relationship between psychedelic use and alcohol use disorder
While clinical trials are investigating the use of psychedelics like LSD in the treatment of alcohol use disorder, anecdotes abound of people who say they lost interest in drinking after taking psychedelics. A new study published in Journal of Psychoactive Drugs examined associations between psychedelic use and alcohol use disorder using data from the National Survey on Drug Use and Health (NDSUH), an annual survey on Americans’ substance use.
The researchers analyzed NSDUH data from 2021, 2022, and 2023 and found that LSD use within the last year and lifetime mescaline and DMT use was associated with lower odds of alcohol use disorder symptoms in the previous year. While previous-year use of MDMA and ketamine showed no correlation with previous-year alcohol use disorder symptoms, lifetime use of MDMA and psilocybin were both associated with a higher likelihood of alcohol use disorder symptoms in the previous year.
Correlational studies like this one reveal only associations between variables, not causation, so it’s unclear whether psychedelic use directly affects alcohol use disorder symptoms. But, the researchers write, this work sheds light on the fact that different psychedelics present different risk profiles in relation to alcohol use disorder symptoms.
Recently, Colorado’s Natural Medicine Advisory Board recommended the state look into adding ibogaine to its program — and to ensure the iboga they import is ethically and sustainably produced in Gabon. But The Colorado Sun reports that a board member filed an anonymous complaint last month asking regulators to investigate Ean Seeb, Colorado Governor Jared Polis’s special advisor on cannabis and psychedelics, for “putting pressure on the board” to change their minds about sourcing sustainable iboga.
In his newsletter Ecstatic Integration, Jules Evans dives into the psychedelic coaching industry, and what accredited coaches think of the practice.
Internal Family Systems, or IFS, is a popular therapy approach, often used in psychedelic-assisted therapy. But some patients tell The Cut that IFS has destroyed their lives.
You’re all caught up! We’ll be back in your inbox on Monday with a new issue of 5 Questions.
If you know anyone who might like the latest on psychedelics in their inbox, feel free to forward this to them, or click below.
Got tips? Email us at themicrodose@berkeley.edu.