Government report recommends that the DEA increase transparency for psychedelic churches, and largest study yet of at-home ketamine effects
Plus: Health Canada grants psilocybin access to cluster headache patient and Arizona governor vetoes psilocybin services
Happy Friday and welcome back to The Microdose, an independent journalism newsletter brought to you by the U.C. Berkeley Center for the Science of Psychedelics.
Government report recommends that the DEA increase transparency for psychedelic churches
The Government Accountability Office (GAO), a federal watchdog agency that audits and investigates the U.S. government, recently published a report that reviewed the use of psilocybin. The report was the result of a line item in the 2023 Consolidated Appropriations Act, an omnibus bill passed by the U.S. Congress defining government spending for each fiscal year. Between February 2023 and May 2024 the GAO collected federal data on enforcement of psilocybin-related laws and interviewed researchers, officials from Oregon and Colorado, representatives from psychedelic advocacy organizations, and employees of federal agencies, including the Drug Enforcement Administration and the Food and Drug Administration. GAO officials even participated in the Psychedelic Science 2023 conference in Denver to attend panels on religious use of psychedelics and to interview experts. Their report addressed two main themes: legal barriers to scientific research using psilocybin and legal barriers to religious use.
The GAO reports that registrations for scientific studies involving psilocybin have increased significantly in the last five years, from 5% of all Schedule I substance research approvals in 2018 to 18% in 2022. (At the time of publication, the agency only had data for the first half of the 2023 fiscal year, when psilocybin accounted for 40% of Schedule I drug approvals for research purposes.) Based on the GAO officials’ interviews with researchers, it is clear that some of the Drug Enforcement Administration’s policies on controlled substances make research difficult. “According to a selected stakeholder from a large university, they must obtain a DEA license for each building included in a psilocybin-related study,” the report states. “The stakeholder said that these steps are redundant and time consuming for a registrant with one study across multiple buildings.” The DEA responded that “this requirement is intended to prevent diversion as the transport of a controlled substance from one location to another can be a security risk.” Researchers also reported to the GAO that communicating with the DEA can be difficult; emails to a general DEA address sometimes went unanswered, and one interviewee said that the DEA would not provide guidance directly to study sponsors.
The report also provides documentation of the long wait times churches have faced in requesting religious exemptions from the DEA for use of controlled substances in their ceremonies. The GAO reports that the DEA received 24 such petitions between 2016 and 2024, but none of them have been granted, and petitioners often wait years for a response. One 2016 petition is still unanswered after eight years; the GAO report says that other churches have formally withdrawn their petitions, or did not respond to the DEA’s requests within 60 days, which the DEA considers a withdrawn petition.
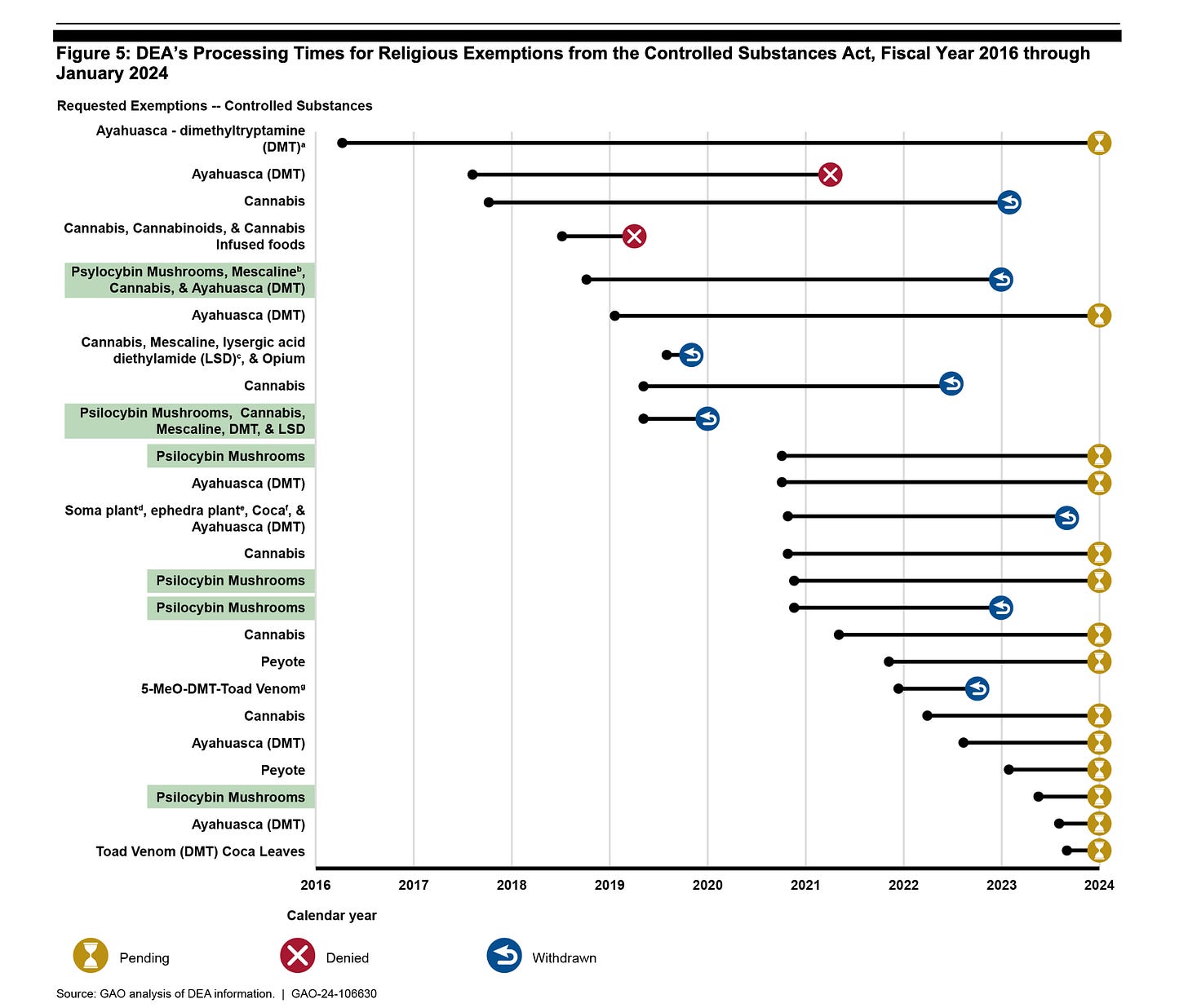
In the GAO report, DEA officials explain that delays can result from a number of issues with petitions, including petitioners’ criminal records, unsafe public safety precautions, or “insufficient information to determine whether a religious belief is sincerely held.” But some petitioners told the GAO that the DEA’s processes and standards for petitions are unclear: “A stakeholder told us that they do not know how much and what type of information DEA would consider to be proof of religious sincerity,” the GAO report says. “Such a requirement can be especially true for those petitioners who come from cultures where psilocybin has been used for generations and do not maintain documentation to prove their use of psilocybin is sincere, according to stakeholders.” Petitioners also told the GAO that the timeline for receiving responses is unknown, which can make it seemingly impossible for churches to operate. The DEA's guidance to petitioners says that it prohibits applicants from using controlled substances "unless the petition has been granted and the petitioner has applied for and received a DEA Certificate of Registration."
The GAO report makes several non-binding recommendations related to churches seeking religious exemptions for their use of a controlled substance. Some of the suggestions are that the DEA administrator should establish timeframes for final decisions on petitions and that the agency should more clearly communicate the types of information petitioners need to provide to prove “religious sincerity.” The report also recommends that the DEA make public the standards it uses in making its decisions about petitions and reliably communicate updates to petitioners on the status of their requests.
Largest study yet of at-home ketamine effects
During the COVID-19 pandemic, the federal government loosened rules about telehealth appointments and prescriptions. One beneficiary of this shift was the growing at-home ketamine therapy industry. Some companies mail ketamine doses directly to patients experiencing anxiety and depression and then provide online “coaching” or “monitoring” during their trips. Some news outlets have described the growing off-label use of ketamine as the “wild west” and have raised concerns that at-home use of the drug could be ineffective, at best, and potentially dangerous. Studies show that regular ketamine use can be associated with symptoms of addiction.
A new paper published in The Journal of Affective Disorders purports to be the largest study yet of at-home ketamine patients. Its authors are affiliated with Johns Hopkins, NYU, a private Miami psychotherapy practice, the Institute for Psycholinguistics and Digital Health, and Mindbloom, a ketamine therapy company. The 11,441 patients in the study were all Mindbloom clients. Participants received four doses of at-home oral ketamine over the course of a month, along with support from a guide via telehealth. They completed mental health questionnaires to assess anxiety, depression, suicidality, and drug and alcohol abuse, as well as any adverse reactions they experienced.
Overall, over 60 percent of participants showed improved depression or anxiety scores after their four ketamine sessions. Around 5 percent reported adverse effects; six people reported serious effects such as psychosis and suicidal behavior that required hospitalization, though most reported milder symptoms, including headache or memory impairment. The study’s authors conclude that Mindbloom’s ketamine administration was “largely safe, well-tolerated, and associated with improvement in patients with depression,” but also acknowledge the study’s limitations, such as a lack of control group to assess placebo effects, the absence of measures of expectancy effects, and whether other types of support or therapy might have contributed to the efficacy of ketamine treatment. Given recent discussion about ketamine abuse and addiction, it’s also interesting to note that Mindbloom clients were given the option of entering a second round of treatment, and that those who elected to do so were more likely to be older, and less likely to live in rural areas or have higher scores on drug and alcohol abuse scales.
In the paper’s declaration of competing interests, the authors — two of whom are not affiliated with Mindbloom — say that while Mindbloom provided data, they “had no involvement in the study design or formal analysis for this manuscript.”
Want the latest psychedelics news? Subscribe! (It’s free!)
Health Canada grants psilocybin access to cluster headache patient
Last week, Health Canada granted emergency access to psilocybin to a Calgary man suffering from cluster headaches. Jody Lance initially applied for access through Health Canada’s Special Access Program in 2023 but was denied. Lance and his neurologist filed a lawsuit, and two weeks ago, a federal court ruled that Health Canada’s denial was “unreasonable” and that the agency would need to reconsider his application. Health Canada complied and reversed their decision last week.
Arizona governor vetoes psilocybin services
On Tuesday, Arizona Governor Katie Hobbs vetoed Senate Bill 1570, which would have created a state-regulated psilocybin services program. The bill was introduced in the State Senate in February. After passing the State Senate four weeks later, it headed to the State House, which passed it 42-16 on Friday. In her veto memo for the bill, Governor Hobbs wrote that “we do not yet have the evidence needed to support widespread clinical expansion,” and that the annual $400,000 necessary to run the program was not accounted for in the state’s 2025 budget. “Arizonans with depression and PTSD deserve access to treatments that may be seen as outside the mainstream, but they should not be the subject of experiments for unproven therapies with a lack of appropriate guardrails,” Governor Hobbs wrote.
In her newsletter Rave New World, drugs journalist Michelle Lhooq interviews a Canadian high school science teacher who’s been making and selling “ayahuasca gummies.” The gummies were designed to mimic ayahuasca by coupling DMT and a MAOI inhibitor, which slows the body’s metabolization of DMT. The gummies offer what Lhooq calls “a fascinating look into how drug decriminalization is opening up space in the underground for some pretty wild kitchen experiments by mommy-chemists.”
This week in Heated, climate reporter Emily Atkin writes about a recent panel put on by Psychedelics for Climate Action, an advocacy group that claims that psychedelics can “inspire people to help solve the climate crisis.” She felt “the event to be lacking some pretty necessary context.”
On X, psychedelics researcher Jacob Aday posted a photograph of a mushroom T-shirt for sale at Walmart.

You’re all caught up! Have a great weekend. We’ll be back in your inbox on Monday with a new issue of 5 Questions.
If you know anyone who might like the latest on psychedelics in their inbox, feel free to forward this to them, or click below.
Got tips? Email us at themicrodose@berkeley.edu.







