Kentucky ibogaine proposal in flux; A look at year one of psilocybin services in Oregon; Drug Enforcement Administration proposes to restrict two new drugs
Plus: DEA increases psychedelic manufacturing quotas for research, Matthew Perry’s death
Happy Friday, happy new year, and welcome back to The Microdose, an independent journalism newsletter brought to you by the U.C. Berkeley Center for the Science of Psychedelics.
The State of Psychedelics: Kentucky ibogaine proposal in flux, California ballot initiative could cost $100 million annually
For the last few months, Kentucky’s Opioid Abatement Advisory Commission has been considering a proposal to use $42 million of its $842 million settlement with opioid manufacturers and distributors to study ibogaine. That commission was helmed by attorney W. Bryan Hubbard, who was appointed by Attorney General Daniel Cameron. Cameron vacated his position to run for governor in November, a race he lost. In late December, newly elected Attorney General Russell Coleman announced his leadership team, which included a new director for the Opioid Abatement Advisory Commission. The man taking Hubbard’s place is Christopher Evans, a former administrator for the Drug Enforcement Agency, according to Kentucky’s PBS station KET. Hubbard was the main driver of the ibogaine proposal, and with his departure, its fate is unclear.
The California Legislative Analyst’s Office published its fiscal analysis of the Psychedelic Wellness and Healing Initiative, which was submitted to the California legislature in early December. The initiative was spearheaded by Dave Hodges, founder of Oakland’s psychedelic Church of Ambrosia, whose Zide Door location was raided by Oakland police in 2020. The initiative includes cannabis as well as psychedelic drugs like psilocybin and LSD, and proposes allowing people over 18 to freely use and cultivate those drugs at home, and for health care practitioners to use them therapeutically. The measure also prohibits the state government from enacting taxes on psychedelic businesses “exceeding the amount assessed for comparable non-entheogenic plant or substance related businesses.” The Legislative Analyst’s breakdown estimates that the decrease in regulatory revenue from reduced cannabis taxes could exceed more than $100 million a year if numerous cannabis businesses were to classify themselves as psychedelic businesses rather than marijuana businesses. While the state could receive additional revenue from psychedelic businesses currently operating underground and not paying taxes, that likely wouldn’t offset the potential loss from cannabis revenues.
The Latest in Oregon: A look at year one
Oregon’s Psilocybin Services (OPS) program has now been up and running for a full year. In that time, the state has licensed 188 new facilitators, 20 service centers, seven manufacturers and two testing labs. OPS Section Manager Angela Allbee told The Microdose that she’s heard that the state’s centers have served at least 600 people. The Healing Advocacy Fund, a nonprofit that supports state-regulated access to psychedelics, says that number is over 700 — because service centers are not yet required to report client data to OPS, those numbers are estimates. But that will change in 2025; on January 1, 2024, an updated set of rules also went into effect, requiring service centers to collect data on their practices and clientele and to report those data to the state.
Want the latest psychedelics news? Subscribe! (It’s free!)
DEA makes second proposal to schedule DOI and DOC
In mid-December, for the second time in recent years, the U.S. Drug Enforcement Administration filed a proposal to classify two previously unscheduled hallucinogens known as DOI and DOC as Schedule I drugs, which the agency defines as having “no currently accepted medical use and a high potential for abuse.” Though there are some records of the drugs being used recreationally, DOI, in particular, is most frequently used in neuroscience research studies.
In April 2022, the DEA published a similar proposal, which came on the heels of another DEA proposal to classify five previously unscheduled tryptamines as Schedule I drugs. Psychedelic activists, researchers, and companies publicly decried both proposals. The DEA scheduled a rare public hearing in the tryptamines case, but before the hearing took place, they quietly withdrew the tryptamine proposal and then withdrew the DOI and DOC proposal as well— an exceptionally rare move from the agency, which has only withdrawn one other Schedule I proposal since it was founded 50 years ago, according to attorney Matt Zorn in his newsletter On Drugs.
What’s noteworthy about the DEA’s latest scheduling attempt is that the new proposal’s only major change is in how it handles requests for hearings; the evidence for scheduling the two drugs remains the same. Previously, anyone could make a request for a hearing, but the DEA’s latest proposal states that requests must be filed along with a “written statement of position on the matters of fact and law asserted in the hearing,” and that the DEA administrator will determine whether the hearing is necessary. This change, writes Zorn, may not be permissible per current DEA regulations, which allow any interested party to request a hearing without having to file a written position statement or allowing an administrator to rule on whether or not the hearing is warranted. The DEA is accepting public comment on the proposal through January 12, and it has already received 95 comments.
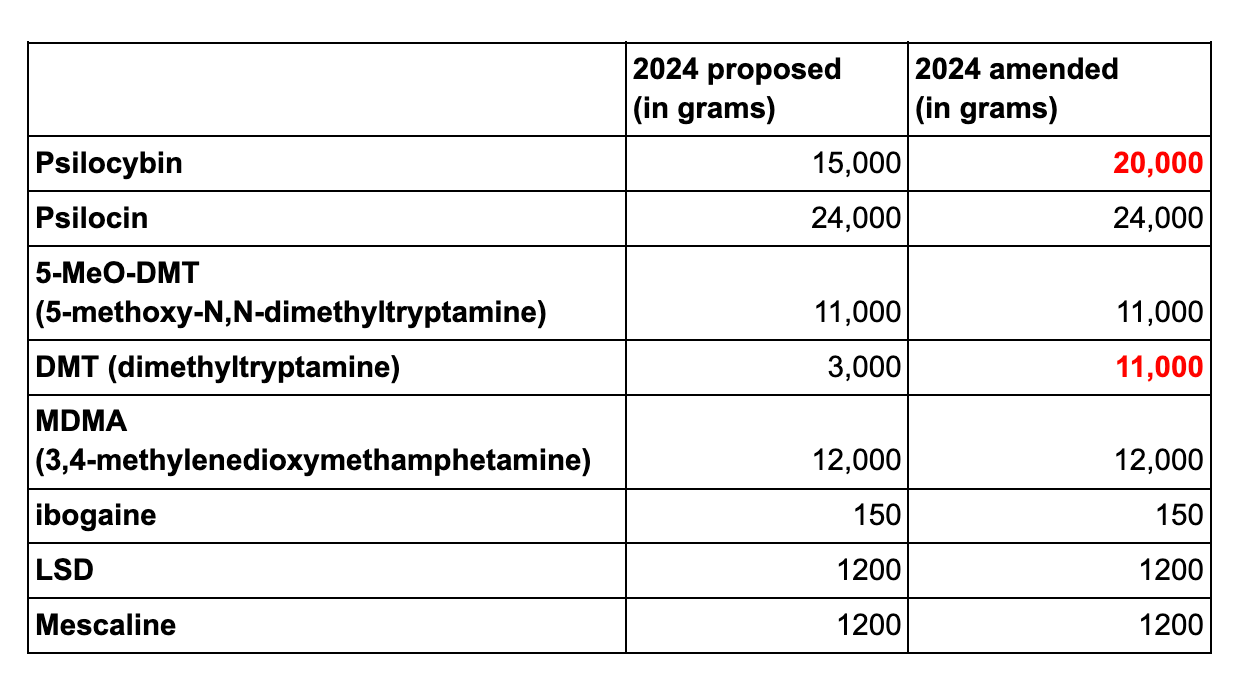
Revised 2024 DEA psychedelic drug quotas
In other DEA news, the agency has increased some of its quotas for the amount of psychedelic drugs manufactured for research purposes in 2024. When the agency first proposed 2024 quotas in November 2023, the amounts it included were the same as its revised 2023 quotas, suggesting the agency expected the amounts of the drugs used in research to remain roughly the same. These revised numbers include a nearly tripled amount of DMT, and a one-third increase in the amount of psilocybin.
Ketamine named as cause of death in actor Matthew Perry’s autopsy
Friends actor Matthew Perry died in late October, and in mid-December, the Los Angeles Department of Medical Examiner released the results of his autopsy. That report attributed his death to the “acute effects of ketamine.” Before his death, Perry was receiving ketamine treatment under supervision of a clinician; his last session was a week and a half before he passed. His autopsy results suggest that he had been taking the drug unsupervised — and that fact has opened a public discussion about the drug’s safety, potential for addiction, and concerns that the high-profile death could compromise people’s ability to access therapeutic ketamine.
Some have called into question whether it’s safe for people to use ketamine at home, unsupervised. In a statement from the American Society of Ketamine Physicians, Psychotherapists, and Practitioners, the organization wrote that “there are some situations in which it may be appropriate for a clinician to prescribe at-home use in between face-to-face appointments” but that their forthcoming best practice guidelines would not recommend telehealth as a model for accessing the drug. “This is a wakeup call for ketamine practitioners and the wider medical community to put clear and unified guardrails in place guided by real-world data and medicine (as opposed to startup profits and flashy business models) in order to protect the people who need this treatment most,” the statement reads.
In a Washington Post op-ed, physician Leana Wen also called for more safety guardrails for the drug. “Just because a substance has legitimate medical purposes doesn’t mean that it’s safe for individuals to consume as a party drug or as unmonitored self-treatment. And conversely, just because ketamine was the culprit in Perry’s devastating death doesn’t mean that patients who would benefit from it should shy away,” she writes. “Ketamine, just like other high-risk drugs, must be approached with caution and rigorous adherence to therapeutic protocol.”
Psychedelics giant atai Life Sciences has invested $50 million in Beckley Psytech, reports Psychedelic Alpha.
In mid-December, Congress passed the National Defense Authorization Act, the Department of Defense’s 2024 fiscal budget, which included funding for the Department of Defense to award grants for psychedelic research.
Business Insider ran an excerpt from Rachel Nuwer’s 2023 book I Feel Love in which she tells the story of how billionaire heiress Elizabeth Koch used MDMA-assisted therapy to heal her childhood trauma and find joy. Her therapy followed the MAPS protocol, and according to Nuwer, Koch is now one of MAPS’s top five donors.
The Economist ran a short profile of outgoing Kentucky Opioid Abatement Advisory Commission director W. Bryan Hubbard and his proposal to use part of the commission’s funds to study ibogaine.
In The Hollywood Reporter, writer James Hibberd shares the details of “the scariest experience” of his life: a session of ketamine therapy.
Print and audio journalists: Apply for a $10,000 fellowship to report stories on psychedelics. Applications and story pitches due 1/31.
You’re all caught up! Have a great weekend. We’ll be back in your inbox on Monday with a new issue of 5 Questions.
If you know anyone who might like the latest on psychedelics in their inbox, feel free to forward this to them, or click below.
Got tips? Email us at themicrodose@berkeley.edu.