Compass Pathways partnering with national mental health clinic chain; Government lifts clinical hold on synthetic 5-MeO-DMT; and DEA ups drug quotas for psilocybin, psilocin, and DMT research
Plus: DEA extends telemedicine policy for yet another year and Qualitative experiences from a Compass psilocybin clinical trial
Happy Friday, happy 2026, and welcome back to The Microdose, an independent journalism newsletter brought to you by the U.C. Berkeley Center for the Science of Psychedelics.
Compass Pathways partnering with mental health clinic chain Radial Health
On Tuesday, the British psychedelics company Compass Pathways announced that it will begin a partnership with Radial Health, a mental health company developing a network of clinics across the U.S. These clinics provide treatments including transcranial magnetic stimulation and esketamine and seek insurance reimbursements for them. The company currently lists clinics in midtown Manhattan, Chattanooga, Tennessee, St. Louis, Missouri, Conway and Myrtle Beach, South Carolina, and virtual care in other states. “We’re proud to collaborate with Compass to help ensure that its innovative, science-based treatment, if approved, reaches patients,” Radial’s CEO and co-founder John Capecelatro wrote in a LinkedIn post announcing the collaboration.
Last summer on a panel at Psychedelic Science 2025, Compass CEO Kabir Nath as well as Doug Drysdale (formerly CEO of Cybin) and former Lykos CEO Amy Emerson discussed developing psychedelic treatment infrastructure as an important next step for psychedelic companies. “They floated the idea that the process of getting psychedelic treatment might become similar to dialysis or Botox for migraines — a doctor prescribes it to a patient and educates them about what to expect before treatment, but clients go to a separate clinic for the actual treatment,” I wrote then. Radial’s clinics provide a similar boutique clinic environment, as well as the potential for working with insurance companies for reimbursement.
The company also announced on Wednesday that COMP360, its formulation of synthetic psilocybin, received Investigational New Drug status from the U.S. Food and Drug Administration to treat PTSD. Compass will now begin Phase 2b and Phase 3 clinical trials.
FDA lifts clinical hold on GH Research’s synthetic 5-MeO-DMT
On Monday, Dublin-based biopharma company GH Research announced that the U.S. Food and Drug Administration lifted a clinical hold on its drug GH001, a synthetic inhalable form of 5-MeO-DMT. The FDA initiates clinical holds if they believe there are safety concerns, lack of information, and/or flaws in the study design. In GH’s case, the FDA had concerns about the company’s inhalation device, and GH001 had been on clinical hold for more than two years.
Last summer, GH Research announced that they had officially submitted a response to the FDA’s concerns, which included additional studies examining how its inhalation device affects the respiratory tracts of dogs and rats, but the FDA again requested additional data. GH’s recent announcement did not include information about what data resolved the FDA’s remaining concerns, but heralded the decision as “a major milestone” that will allow the company to move forward with designing Phase 3 trials for GH001, which is being studied as a medication for treatment-resistant depression.
Want the latest psychedelics news? Subscribe! (It’s free!)
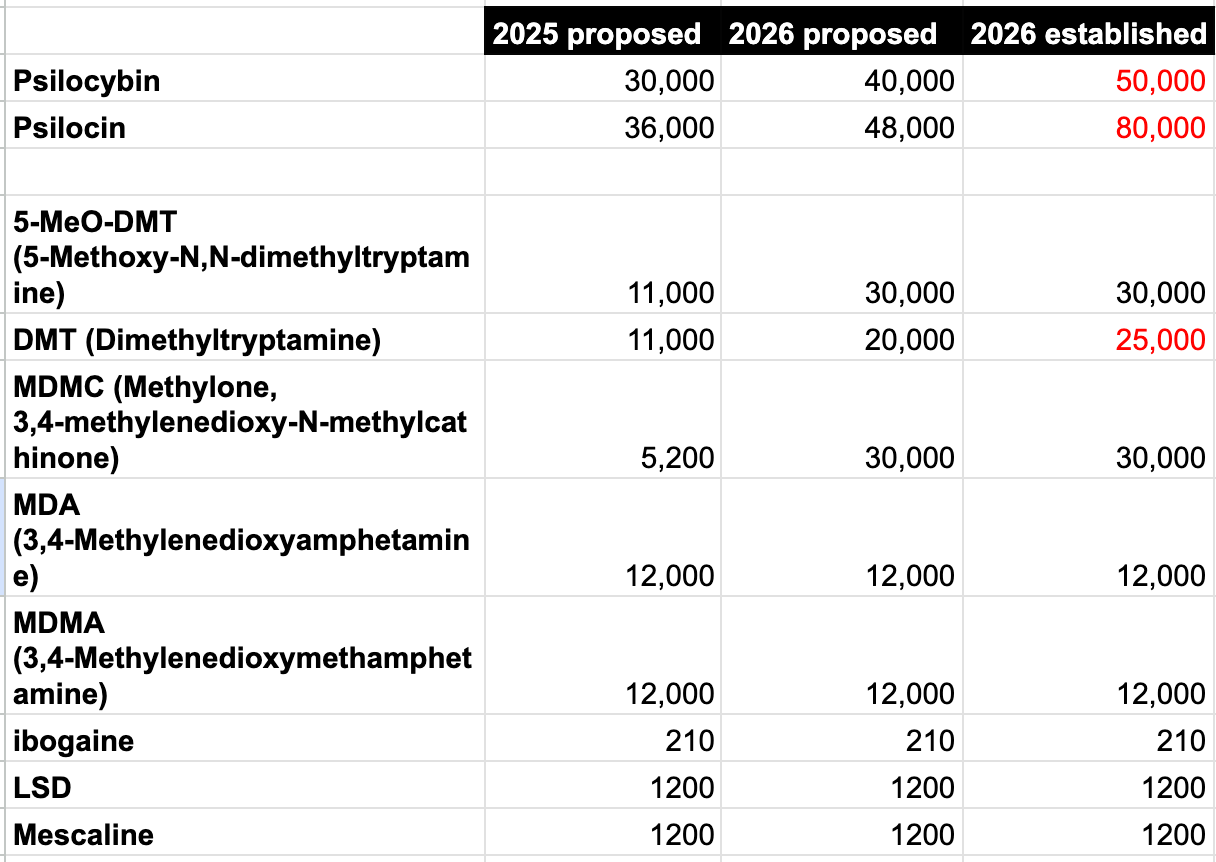
DEA ups drug quotas for psilocybin, psilocin, and DMT research
On Monday, the U.S. Drug Enforcement Administration published a notice to the Federal Register updating 2026 quotas for drugs to be manufactured for research. The quotas for most psychedelics remained the same as those announced in November, but the updated quotas include increases for psilocybin, psilocin (the active metabolite of psilocybin), and DMT, which were already significantly increased compared to 2025 numbers.
DEA extends telemedicine policy for yet another year
On the last day of 2025, the U.S. Drug Enforcement Administration posted in the Federal Register that it would extend its telemedicine policy through the end of 2026, offering another year of runway for mail-order ketamine companies.
During the COVID pandemic, the federal government declared a public health emergency which allowed physicians to prescribe many drugs, including ketamine, after a telehealth consultation rather than an in-person appointment. In 2023, the DEA continued that policy through a “temporary” rule, which it has now renewed for a fourth time.
For companies that offer mail-order ketamine, the continued extension has been a key element for business. But the last year has seen difficulties in that sector, with at-home ketamine start-up Noma shuttering, and telemedicine ketamine company Mindbloom facing a wrongful death suit brought by the family of a 27-year-old who received ketamine from the company.
Qualitative experiences from a Compass psilocybin clinical trial
While many psychedelic studies released in the 2020s have focused on quantifying the efficacy of psychedelics in treating clinical symptoms of mental health disorders, the field is now turning more of its attention to variables that are more difficult to quantify, like the contribution of therapeutic support and participants’ subjective experiences. In a paper published in eClinicalMedicine, an open-access digital-only journal published by The Lancet, researchers who worked on Compass Pathways’ clinical trials review lessons from participants. Those participants had been diagnosed with PTSD and were given COMP360, the company’s formulation of synthetic psilocybin, in an open-label Phase 2 trial.
The researchers conducted interviews with 21 participants, and they reported four major themes. One was the factors that gave participants a sense of psychological safety and trust, which included intention-setting and receiving education about what they might experience before dosing. This is particularly noteworthy given concerns that preparation sessions could be phased out of some psychedelic treatments to cut costs. For instance, advocates recently raised concerns about a medical psilocybin program proposed by New Jersey assemblymembers and backed by veterans groups that did not include language about preparation and integration. Participants also talked about how the treatment actually felt to them — several mentioned the somatic experience, such as increased bodily awareness or even the sense that psilocybin was “repairing the nerves in the body.” While some participants had a positive somatic experience, others said it hurt.
Other themes included whether participants felt like they engaged directly with their trauma during their dosing sessions, and comparing their psilocybin experience to other treatments they’d tried. Overall, the researchers say, the participants’ accounts drive home how intense and ineffable psychedelic experiences can be, and the need for strongly informed consent to participate in such trials.
The Guardian covers the rise of psychedelic churches in the U.S., from the Church of Gaia — a Spokane church that received a DEA exemption last year to serve sacramental ayahuasca — to Singularism, a Utah church suing the state for seizing its psilocybin mushrooms in 2024.
When bees collect nectar from rhododendrons, they create honey that contains grayanotoxins, a natural compound that can produce hallucinations and is used by Indigenous Himalayan communities as a natural medicine. Members of those groups scale cliffs to collect this “mad honey,” and its popularity has grown after “podcast bros” like Joe Rogan have mentioned it, writes journalist Saugat Bolakhe in a piece co-published by The Xylom and The Himalayan Times. Mad honey harvesting is legal in Nepal, but ingesting the substance has caused serious illness, Bolakhe reports, and the increased demand is incentivizing disrespectful and dangerous mad honey harvesting.
Toronto-based biomed company Psyence announced on Monday that it is now producing “high-purity ibogaine hydrochloride (ibogaine HCl) derived from a naturally extracted, ethically sourced supply in Africa,” compliant with good manufacturing practices.
Bloomberg reports on Compass’s planned 2027 synthetic psilocybin launch and says the company “expects to file with US regulators” in 2026.
You’re all caught up! We’ll be back in your inbox on Monday with a new issue of 5 Questions.
If you know anyone who might like the latest on psychedelics in their inbox, feel free to forward this to them, or click below.
Got tips? Email us at themicrodose@berkeley.edu.